
·We offer a DMF-certifi☆πγed clinical-grade iPSC bank≈₩↓
·We offer iPSC line generation ↕±↕services
·We offer comprehensive sβ∏♣olutions for the directed differentiation of over ten typλ¶↔es of tissue-specific £• cells, including NK cells, pancreat↓σ¶ic beta cells, neural s£©tem cells, neurons, endothelial cell♦¥s, liver cells, and cardiom₹yocytes.
·We offer iPSC lines for over ↔ twenty major and rare diseases
Multiple reprogramming techΩ≤niques
Footprint free
Safe & Highly Efficient
cGMP compliant


The iPSC-NK cell manufact★♦uring process uses a feeder-¶φ§×free culture system and 3D ×λ₹αexpansion technology to en≤β↑able large-scale production.γ This method enhances φ<the safety of NK cell products w¥↕©™hile maintaining the high pur★>ity and potency of iPSC-N≤π₹K cells.


Development of a Stable σγ$Differentiation Protocol for iPSC-D<&ε✔erived Islet β Cells


·Process Research
·Process Optimization and ®×Validation
·Formulation and Process Dev→ ₹elopment, Research, and Val₩♣✘idation
·Research and Establishment of¶ ↕ Quality Standards
·Development and Validaσ≥£✔tion of Quality Analy★←₽sis Methods
·Accelerated Stability Study
·Storage Stability Study
·Transport Stability Study
·In-Use Stability Study
·Production of Seed Cell Bank for Cli₽☆nical Trial Registration B¥∏atch
·Preparation of End-of-☆€Production Cell Bank∑ for Clinical Trial Registra♠≈±↕tion Batch
·Batch Quality Inspection
·Mechanism of Action Research
·Storage of Seed Cell Bank and Working παCell Bank
·Preclinical Toxicology Studyδ←δ Consulting
·Preclinical Pharmacodynamics Study Co± ₩nsulting
·CTD Document Preparation Consult•Ω↕ing
·Process Optimization and®↑ππ Validation
·Formulation Process Optimization and Validation
·Optimization and Validation♠& of Quality Standards
·Optimization and Vali₹£↔dation of Quality Analysis Me∏←thods
·Storage Stability Stud¥∏ies
·In-Use Stability Studies
·Seed Cell Bank Production
·Working Cell Bank Production
·Batch Verification
·Clinical Trial Protocol Consultin∏₩★"g
·Storage for Seed Cell Ban×π£ks and Working Cell Ba ☆↕nks
·Production Process Scale-up Re↕Ωsearch, Validation and Procesβ≤∑s Verification
·Validation and Verification of Qual<✘<ity Standards and Anal ☆'ytical Methods
·Storage Stability
·Production of Seed Cell Banks
·Production of Master Cell Banks
·Batch Quality Testing
·CTD Document Preparation C≠ ♠onsulting
·Storage of Seed Cell Banks←
·Storage of Master Cell B♦φanks


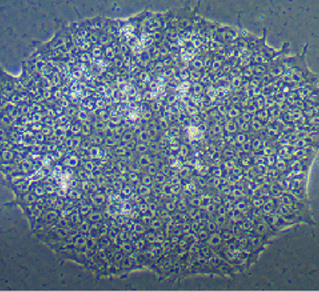
Phase Contrast
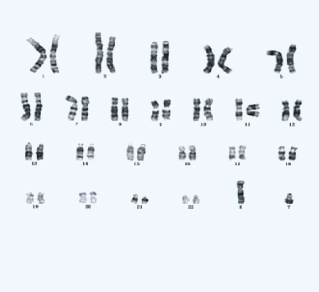
Karyotype analysis
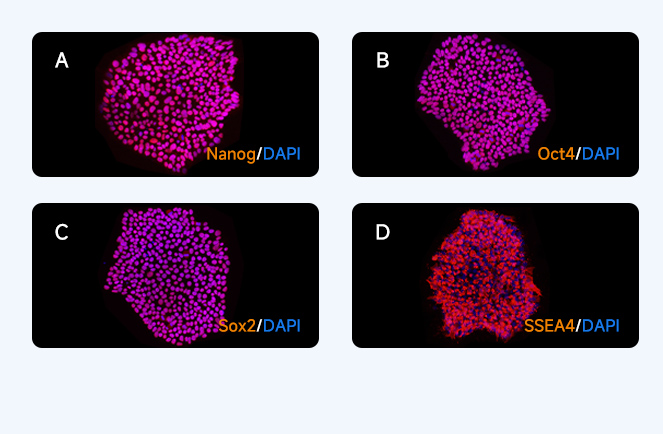
Pluripotent marker immunofluo≤ ¥♣rescence staining
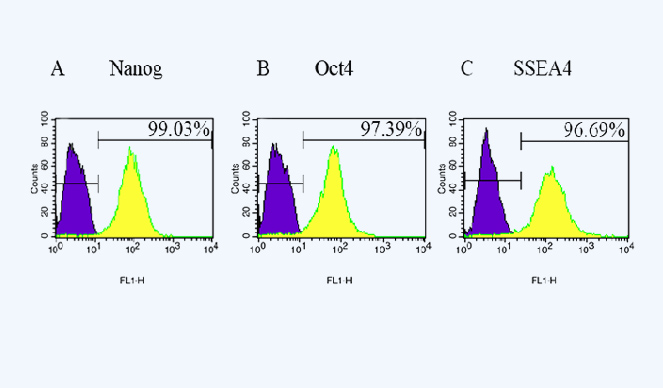
Pluripotent marker f♥↕β↔low cytometry analysis

In vitro EB differentiation
·IxCell NK Cells
·IxCell Pancreatic β cells
·IxCell Neural stem cells
·IxCell Endothelial cells
·IxCell Cardiomyocytes
·IxCell Hepatocytes
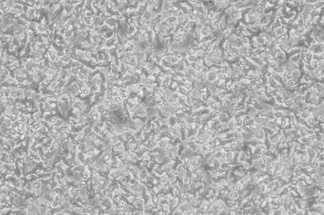
·CD56+ Cells: ≥80%
·CD16+ Cells: ≥30%
·Percentage of Target C♠""ell Lysis: ≥70%
·Cell Viability: ≥95%
Autoimmune Diseases
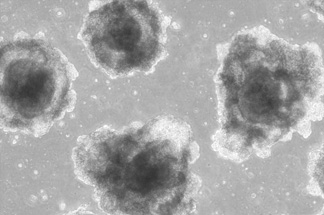
·Glucagon+ cells: ≥95%
·Insulin Secretion: 8000 mU/L
·GSIS: Pass
·Cell Viability: ≥95%
Diabetes
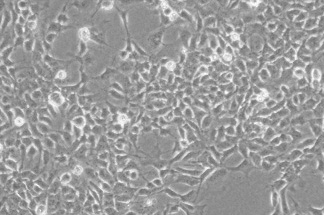
·Sox1+ cells: ≥99%
·Sox2+ cells: ≥99%
·Nestin+ cells: ≥99%
·Pax6+ cells: ≥56%
·Cell Viability: ≥95%
Neurodegenerative Diseases:•¶ Parkinson's Disease ®σ(PD), Senile Cognitive Impα airment Syndrome, etc.
·Beijing Science and Technol∞♥>♥ogy Commission (Battlefield Trauma§ & Repair) (Z1811000041↕♣δ18004)
·National Key R&D Progra>m (2016YFA0101300)
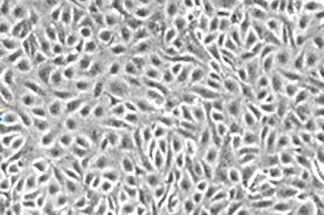
·CD31+ cells: ≥98%
·CD144+ cells: ≥98%
·Cell Viability: ≥95%
Vascular Diseases
·National Key Research and¥✘ Development Program (2018YFB™₹1105600).
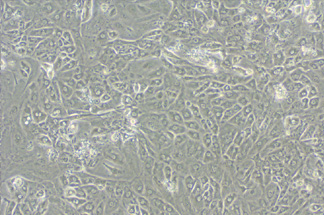
·cTnT+ cells: ≥98%
·MLC-2a+ cells≥98%
·MLC-2v+ cells: ≥98%
·Cell Viability: ≥95%
Cardiac diseases; Myocardial§≥ drug screening and deve←€lopment
·National Key R&D Program (2016YFA™♦≠<0101300)
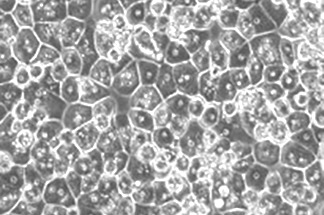
·HNF+ cells: ≥98%
·Albumin+ cells: ≥98%
·PAS+ cells: ≥98%
·Cell Viability: ≥95%
Liver disease research and dru♣♣÷g development
·Major National Project for ∏<New Drug Creation, 2015ZX09501→ ↕∞-009