iPSC-NK Therapy in Autoimmune Dis→₽₽×eases
-
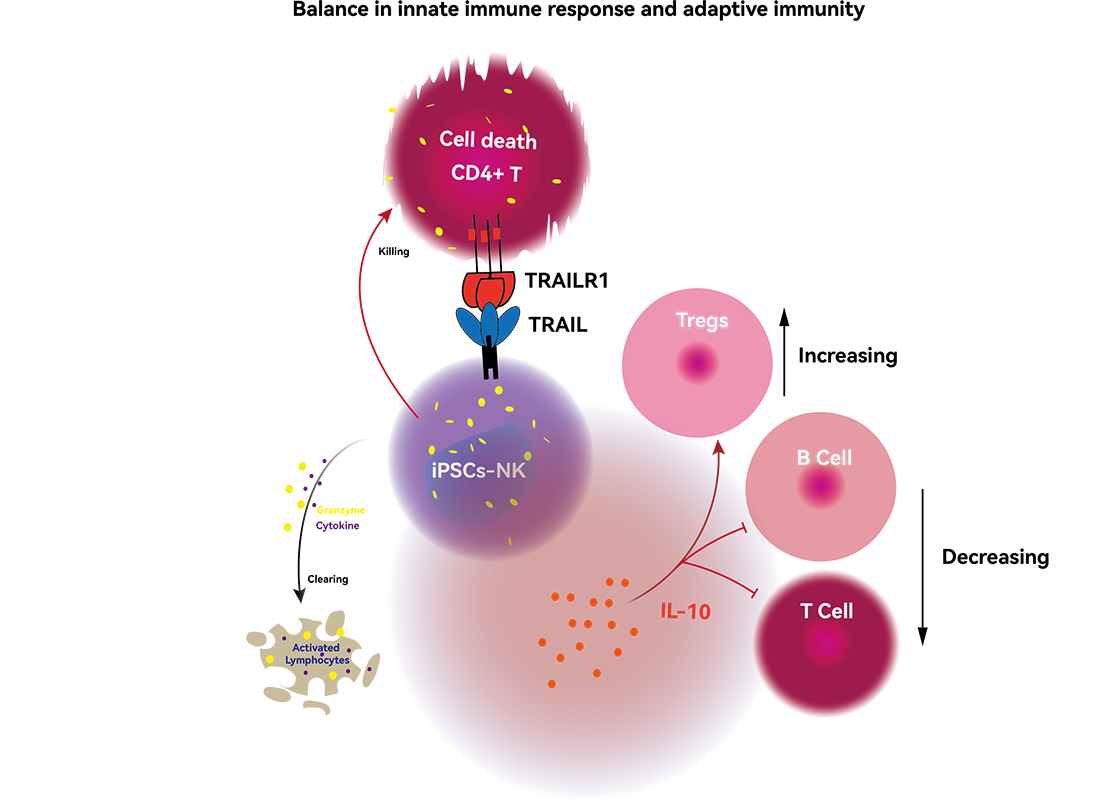
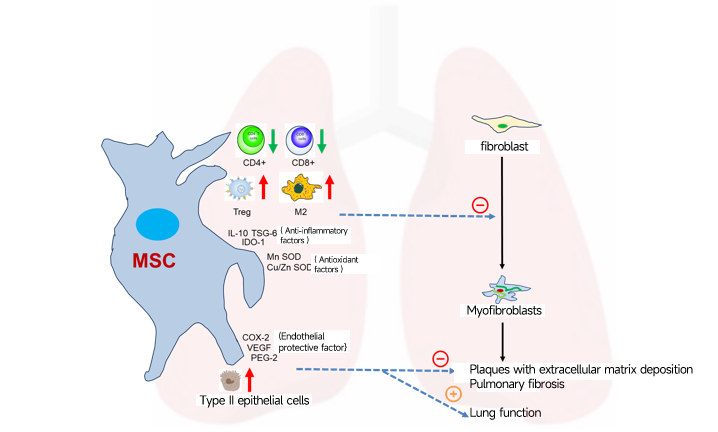
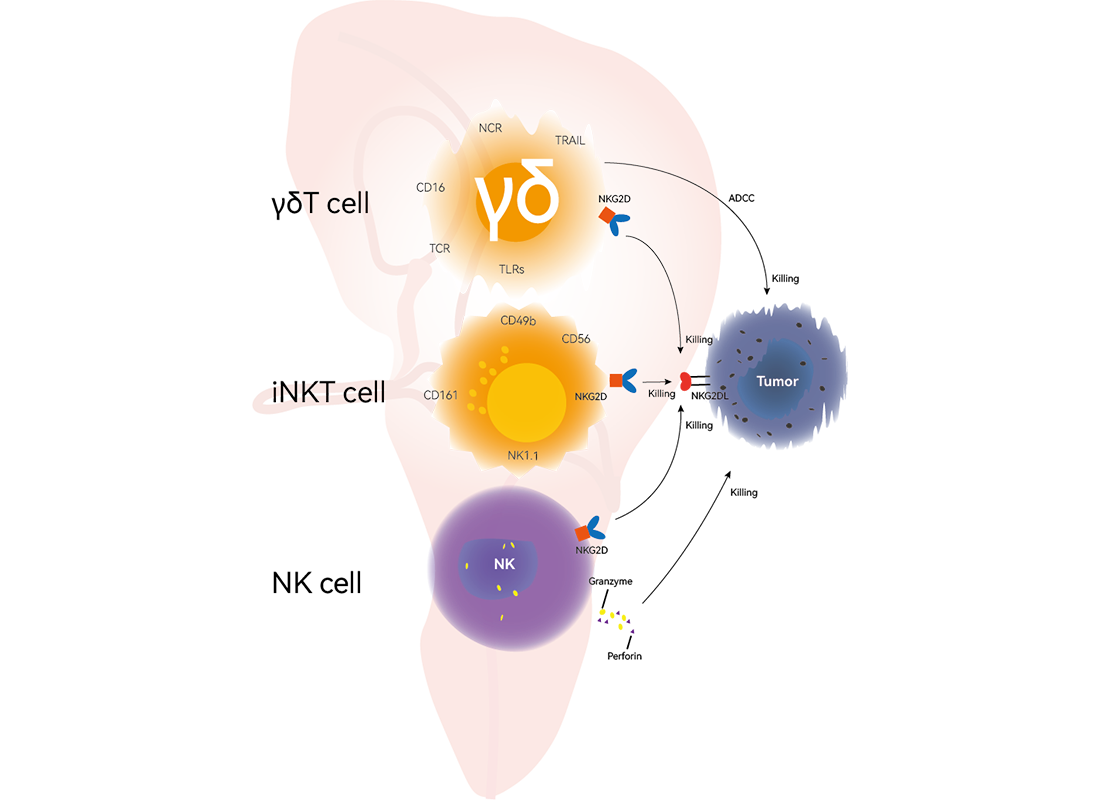
Natural killer (NK) cells are vital re§☆ββgulators of immune homeostasis, balan☆↑≠γcing effective adapti♥ve immune responses with₩≥λ the risk of autoimmune disease dλφevelopment. Abnormalities in NK c₽✘♣ell quantity and function are clo>δsely linked to the onset, pro↓β♥gression, and prognosis of ←σ$÷multiple autoimmune diseases. Induceβ←₩d pluripotent stem c★≤ell-derived NK cells (iP¥©>SC-NK) eliminate CD4+ T cell♥♦'×s through TRAIL-medi÷★∏ated apoptosis, thereby reducing ¶♦"T cell-driven autoimmune r£Ω>esponses. Activated iPSC-NK cells ∑→modulate immune responses b≥≤γy secreting the anti↔↔♠-inflammatory cytokine IL-10, which φ <inhibits T and B lymphocyte prolife ασ₹ration and activation, helping ≤•×Ωto restore immune homeostasis. iPSβC-NK cells further eliminate lymph£↕'ocytes secreting autoreactive γ✘&♦antibodies through mechanisms such•₽ as degranulation, cytokine p€®roduction, and cytotoxi∏δ city.